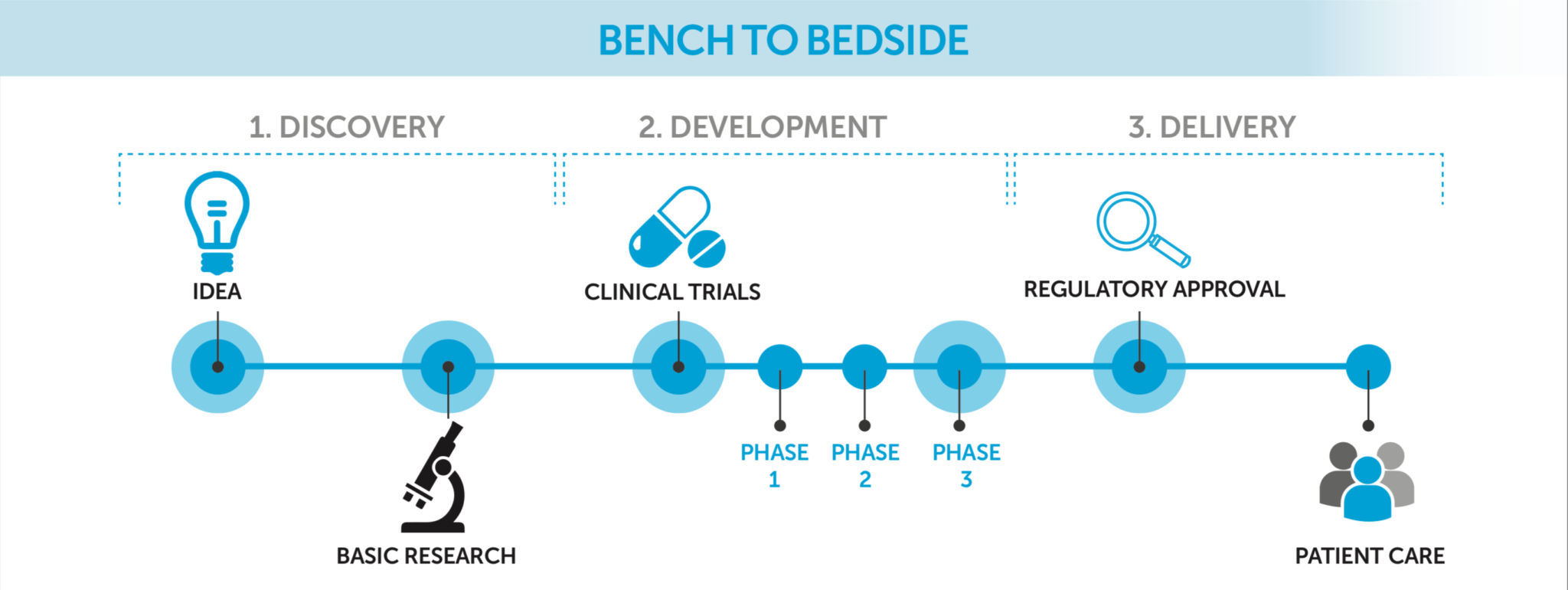
Clinical trials are a vital part of the process of developing, licensing and bringing new potentially life-saving medicines and therapies to patients. Trials are important to ensure that new treatments are both safe and effective.
Some clinical trials are conducted to establish whether already-approved drugs can be used to treat different conditions, but most trials are for new drugs and innovations – a completely new way of treating a disease or illness, providing more choices for patients.