Synthetic Biology and Drug Discovery (Theme Head - Synthetic Biology)
Professor Oliver Rackham

Synthetic Biology and Drug Discovery (Theme Head - Synthetic Biology)
Professor Oliver Rackham
Profile
Professor Rackham’s team focuses on using synthetic biology to engineer and understand transcriptomes, the genetic code, genomes, and organelles. Oliver established his own group at the Perkins in 2006 as an National Health and Medical Research Council (NHMRC) Peter Doherty Fellow. Since then he has been awarded a Wenner-Gren Foundation Fellowship, an ARC Future Fellowship, the Marshall Medal, and was inducted into the European Inventor Hall of Fame. He was a founding member and past President of Synthetic Biology Australasia. Oliver is currently an NHMRC Senior Research Fellow and Node and Theme Leader of the ARC Centre of Excellence in Synthetic Biology.
Research Overview
Professor Rackham‘s research falls into two areas of interest: engineering and understanding mammalian gene expression, and synthetic biology. His work focuses on developing new tools and therapeutics to target cancer, mitochondrial diseases and antibiotic-resistant bacteria.
Research Projects
Synthetic Biology
One of the key aims of synthetic biology is to program cells with new functions. To achieve this aim it is necessary to create additional, new components that interact in a programmable manner, both with each other and with the existing cellular network. To engineer these components we have created a number of powerful new genetic selection approaches that can be used to tailor the molecular specificities of genes, RNAs and proteins in bacteria and yeast. Current projects involve developing new and improved gene editing systems and engineering cells to produce new antibiotics.
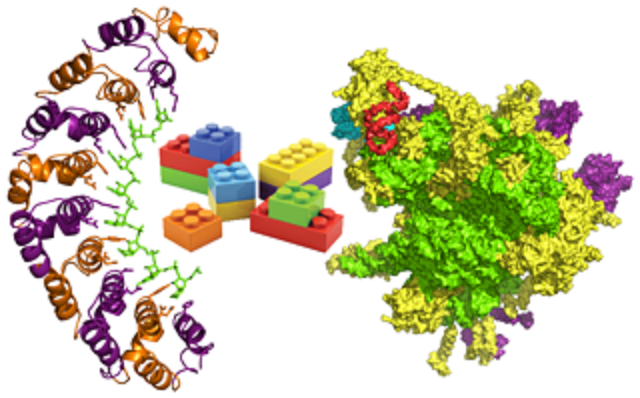
Mammalian Gene Expression
From synthesis to destruction, mRNAs are associated with an array of proteins. Proteins control the efficiency of transcription, processing, nuclear export, translation, localization and degradation of mRNA. The importance of regulation at the level of mRNA has become increasingly apparent with the discovery of disease causing defects in these processes. We are using synthetic biology and transcriptomic approaches to engineer and understand mammalian RNA-binding proteins for use as tools in biotechnology and as therapeutics for human diseases.
Selected Publications
Saurer M, Leibundgut M, Nadimpalli HP, Scaiola A, Schönhut T, Lee RG, Siira SJ, Rackham O, Dreos R, Lenarčič T, Kummer E, Gatfield D, Filipovska A, Ban N. (2023) Molecular basis of translation termination at noncanonical stop codons in human mitochondria. Science 380(6644):531-536.
Hughes LA, Rudler DL, Raven SA, Peng L, Moh ESX, McCubbin T, Siira SJ, Brown JM, Ermer JA, Rientjes J, Rodger J, Marcellin E, Rackham O*, Filipovska A*. (2023) Copy number variation in tRNA isodecoder genes impairs mammalian development and balanced translation. Nature Communications 14:2210.
Vos PD, Rossetti G, Mantegna JL, Siira SJ, Gandadireja AP, Bruce M, Raven SA, Khersonsky O, Fleishman SJ, Filipovska A, Rackham O. (2022) Computationally designed hyperactive Cas9 enzymes. Nature Communications 13(1):3023.
Raven SA, Payne B, Bruce M, Filipovska A, Rackham O. (2022) In silico evolution of nucleic acid-binding proteins from a nonfunctional scaffold. Nature Chemical Biology 18(4):403-411.
Ferreira N, Perks KL, Rossetti G, Rudler DL, Hughes L, Ermer JA, Scott LH, Kuznetsova I, Richman TR, Narayana VK, Abudulai LN, Shearwood AJ, Szappanos HC, Tull D, Yeoh GC, Hool LC, Filipovska A, Rackham O. (2019) Stress signaling and cellular proliferation reverse the effects of mitochondrial mistranslation. EMBO Journal e102155.
Kummer E, Leibundgut MA, Rackham O, Lee RG, Boehringer D, Filipovska A, Ban N. (2018) Unique features of mitochondrial translation initiation revealed by cryo-EM. Nature 560:263-267.
Spåhr H, Chia T, Lingford JP, Siira SJ, Cohen SB, Filipovska A, Rackham O. (2018) Modular ssDNA binding and inhibition of telomerase activity by designer PPR proteins. Nature Communications 9(1):2212.
Siira S, Spåhr H, Shearwood AJ, Ruzzenente B, Larsson NG, Rackham O*, Filipovska A*. (2017) LRPPRC-mediated folding of the mitochondrial transcriptome. Nature Communications 8(1):1532.
Coquille S, Filipovska A, Chia T, Rajappa L, Lingford JP, Razif MF, Thore S, Rackham O. (2014) An artificial PPR scaffold for programmable RNA recognition. Nature Communications 5:5729.
Thyer R, Filipovska A, Rackham O. (2013) Engineered rRNA enhances the efficiency of selenocysteine incorporation during translation. Journal of the American Chemical Society 135(1):2-5.
Filipovska A, Razif MF, Nygård KK, Rackham O. (2011) A universal code for RNA recognition by PUF proteins. Nature Chemical Biology 7(7): 425-427.
Rackham O, Wang K, Chin JW. (2006) Functional epitopes at the ribosome subunit interface. Nature Chemical Biology 2(5): 254-258.
Rackham O, Chin JW. (2005) Cellular logic with orthogonal ribosomes. Journal of the American Chemical Society 127(50): 17584-17585.
Rackham O, Chin JW. (2005) A network of orthogonal ribosome•mRNA pairs. Nature Chemical Biology 1(3): 159-166.
